Why this medical device?
I am the Principal Investigator and Researcher for a clinical trial of a new medical device that is expected to be used in Rwanda and other African countries in the near future. This website was created to give information related to the new device and to provide enough information to participants of the clinical trial.
The clinical trial and the development of the medical device is sponsored by Spiker Ltd., a Japanese health technology company and the device implemented by a multi-national and interdisciplinary team from around the world.
The clinical trial aims to evaluate the performance of a novel intelligent and Artificial Intelligence (AI) powered clinical decision support (CDS) system that was recently developed by Spiker Ltd. The results of the clinical trial will be used in acquiring national and international certification before the device is mass-produced and distributed in Africa in general, and in Rwanda in particular.
The device is fully functional. The protocols of the clinical trial of this devices has been approved by the Rwanda National Ethic Committee (Ethical Clearance No 29/RNEC/2022) and the clinical trial further approved by the Rwanda Food and Drug Administration (FDA) to be installed and tested in Rwandan hospitals.
WHY THE DEVICE
Intrapartum hypoxia (i.e., oxygen deficiency during birth)—commonly known as birth asphyxia— emerges before, during, or after birth when a baby does not receive an adequate amount of oxygen. Intrapartum-related disorders are responsible for 25% of the world’s neonatal death and contribute to almost 50% of the 2.6 million third trimester stillbirths and are a significant burden to healthcare in low-income countries. In 2010, for example, 1.15 million infants developed neonatal brain damage due to some intrapartum hypoxic events, 90% of these complications taking place in low and middle-income countries.
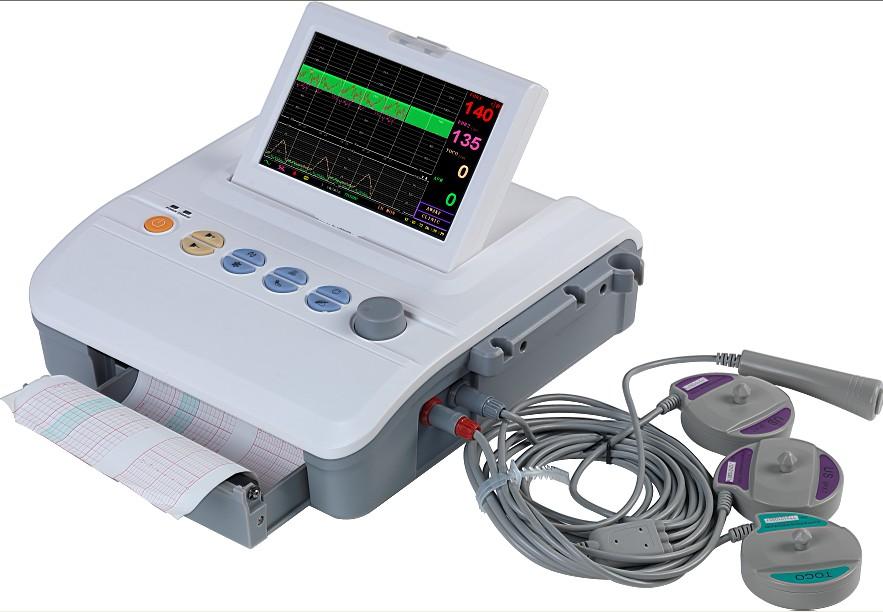
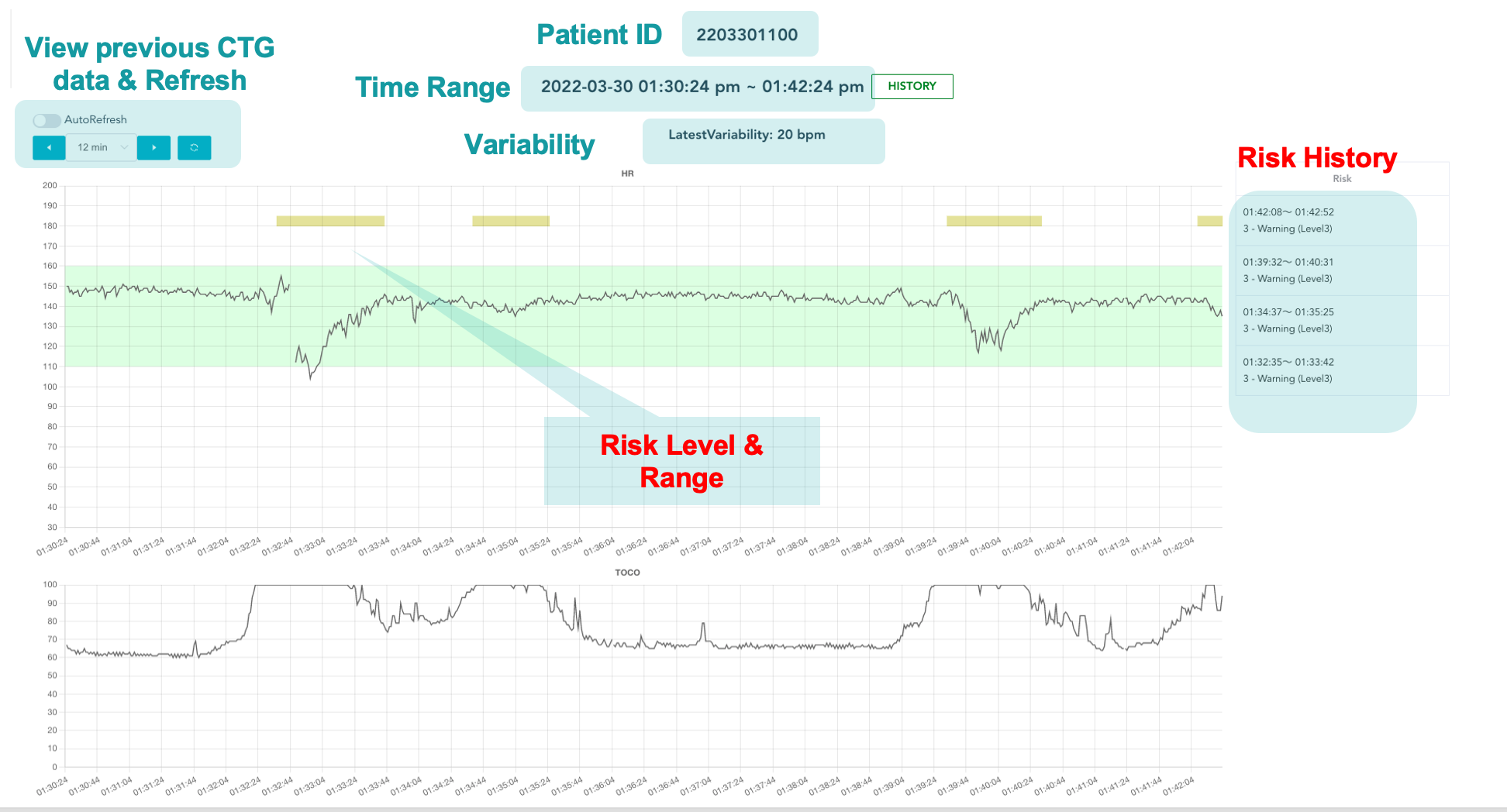
In most hospital in Rwanda, intrapartum cardiotocography (CTG) is often used during childbirth to monitor the heart rate of the baby and the mother’s uterine contractions. A CTG graph can help to evaluate the baby’s well-being and identify babies at risk of hypoxia during labor and to decide whether the baby will be delivered by instrumental vaginal birth or by cesarean section.
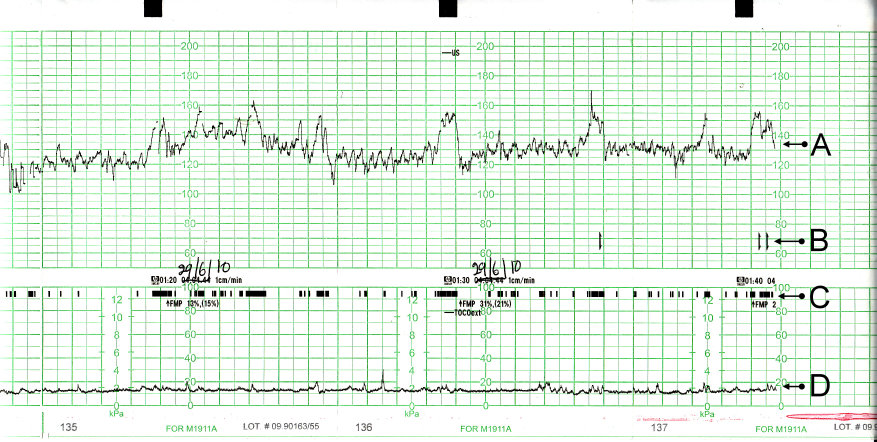
A CTG trace calculate heart rate from fetal heart motion determined by ultrasound, and uterine contractions are measured by a tocodynamometer. In this figure, (A) represents the fetal heartbeat, the (B) indicator shows the movements felt by mother (triggered by pressing a button), (C) shows the fetal movement and (D) show the uterine contractions
During childbirth, obstetricians visually examine the morphological variations in CTG traces to identify the health status of a fetus. This approach, however, is unreliable and has poor reproducibility because the interpretation is often subjective and varies from one obstetrician to another. When there is a misinterpretations, out of an abundance of caution, obstetricians often perform unnecessary operative deliveries. Moreover, the fetus’s heart rate variability is influenced by the brain’s parasympathetic and sympathetic activities and its patterns are affected by several other stimuli such as the uterine contractions. Consequently, intrapartum fetal monitoring must also consider a physiological viewpoint instead of just focusing on the morphological appearance of the fetal heart rate. CTG traces contain complex information about the baby’s condition and identification of the baby’s hypoxemia (e.g., low blood oxygen). Thus, proper CTG interpretation would require the medical personnel to have a good understanding of the physiology of the fetus during childbirth and other compensatory mechanisms of the fetus. Visually analyzing CTG traces cannot reveal such important diagnostic information and its appropriate interpretation.
Finally, several human factors such as distraction, tiredness, pressure, and stress complicate CTG interpretation and may lead to fatal outcomes. For example, the Royal College of Obstetricians and Gynecologists’ report on perinatal mortality in the UK shows that 66% of stillbirths, neonatal deaths, and brain injuries of term babies in labor in 2017 are due to errors of interpretation of CTG and failure to act upon suspicious or pathological CTG. In several cases, when there is an ambiguity in CTG interpretation, obstetricians often resort to unnecessary emergency cesarean sections instead of waiting for a natural vaginal birth.
EXPECTED IMPACT OF THE DEVICE
The device is expected to provide the following impacts:
To reduce the time and training required to properly interpret a CTG graph
The device is designed to automatically streamline operations and CTG measures (e.g., risk definition, contractions, baseline rate, variability, accelerations, decelerations, and overall impression) that are required to properly interpret a CTG graph. In our test, it was shown that it takes less than 1 days for a novice medical personnel to learn how to independently interpret a CTG when he/she used our device.
Reduction of unnecessary cesarean section
Poor CTG interpretation leads to a high false-positive rate of pathological CTG, which, out of an abundance of caution, obstetricians perform unnecessary emergency intrapartum cesarean sections for fetal distress. Recent studies show that proper CTG interpretation and its use in conjunction with fetal ECG ST segment analysis (STAN) lead to a statistically significant reduction of intrapartum emergency cesarean section and the rate of hypoxic-ischaemic encephalopathy (HIE) due to CTG misinterpretation can be reduced by 50%. In our pilot test, it was found that the device achieved more than 3,000 cases of APGAR (Appearance, Pulse, Grimace, Activity, and Respiration) score greater than 8, and no unnecessary cesarean section was performed when using this device.
Reduce risks of neonatal seizures
Neonatal seizures are sudden abnormal modifications of a baby’s electrographic activity during the neonatal period. It is estimated that neonatal seizures occur in 3 per 1000 live births and are much higher in preterm infants. Neonatal seizure is particularly high in rural low and middle-income countries. For instance, in Kenya’s rural districts, neonatal seizures are associated with a 22.5% mortality rate, which is much higher compared to developed countries. Studies conducted around the world show that continuous use of CTG monitoring in labor halved the risk of neonatal seizures, and this reduction is consistent across several subgroups.
To reduce birth asphyxia
Birth asphyxia (i.e., the insufficient flow of blood or oxygen exchange to or/and from the fetus before, during, or after birth) affects 23% of births worldwide and up to 39% of births at district hospitals in Rwanda. Birth asphyxia is associated with cerebral palsy and long-term impairments such as learning difficulties. The developed device will provide a rapid and accurate interpretation of a cardiotocograph (CTG) during childbirth; thus, allowing rapid identification of pathological CTG that might be associated with birth hypoxia and asphyxia. This will allow the medical personnel to take timely action and appropriate to prevent birth asphyxia.